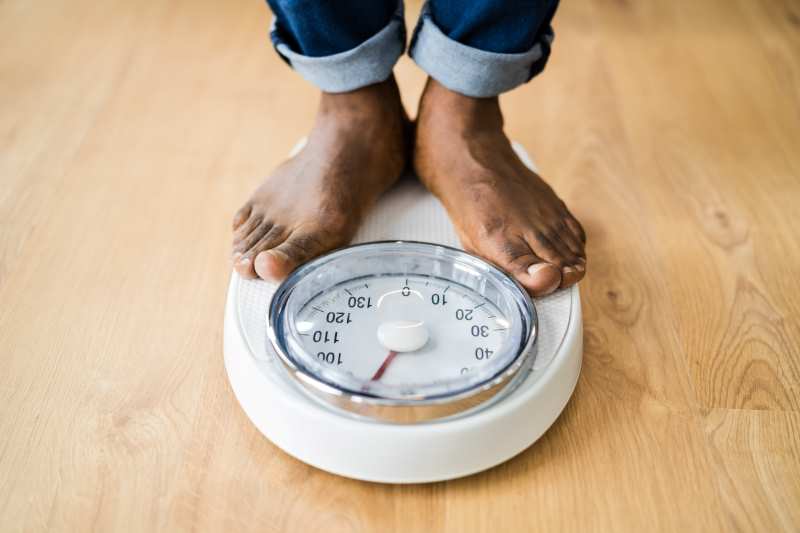
Weight management in older adults requires a fundamental shift from traditional weight-loss approaches.
Obesity and Health Disparities
Advertisement
The results support that circadian misalignment, rather than meal-to-sleep interval, underlies the adverse glucose effects.
Dr. Cory Rice talks about helping patients after they've stopped GLP-1 treatment but still have cardiometabolic challenges.
Between 1990 and 2022, obesity rates quadrupled among girls (from 1.7% to 6.9%) and among boys (from 2.1% to 9.3%) globally.
Dr. Cory Rice discusses alternatives for patients who stop GLP-1s but still need help with disease reversal.
This work represents an important step forward in understanding the complex relationship between socioeconomic status
High fructose intake and altered sugar metabolism in the brain might contribute to neuroinflammation, amyloid beta production
The commission urges health authorities to recognize obesity as a chronic, potentially life-threatening condition
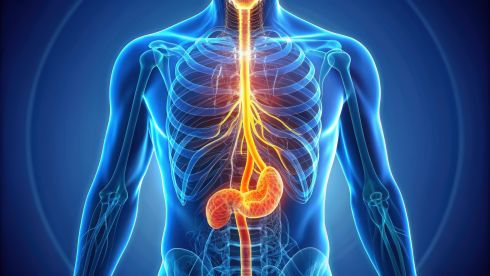
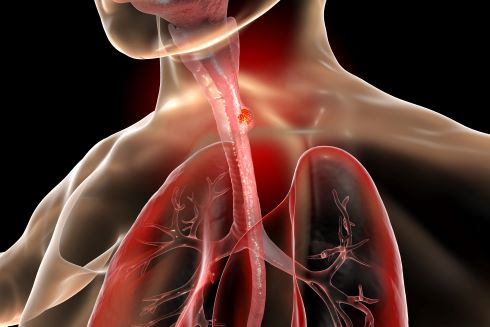
Studies show that obesity is a risk factor for both Barrett's esophagus and esophageal cancer.
Drs. Julie Schulz and Michael Dovidio Discuss Using Lab Values for GLP-1 Drug Coverage.
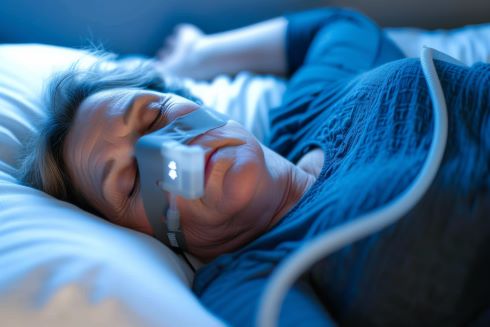
the potential to narrow the gap in sleep apnea outcomes that exists between well-resourced communities and those that have lo
Obesity significantly increases the risk of multiple myeloma, especially in Black populations.
People who consume diets rich in ultra-processed foods (UPF) have a 26% higher risk of dying from COPD.
Gastric bypass leads to significant and sustained weight loss and improvements in hypercholesterolemia over 11 years.
If those individuals also have a higher risk of colorectal cancer, we can infer that obesity is linked to colorectal cancer.
Urban Health Today reports on clinical news and policy updates that directly impact urban health care providers and their patients.
Get research and expert insights straight to your inbox.










 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.